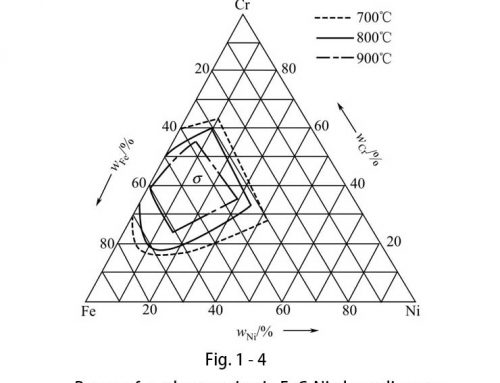
Effects of carbide-forming elements
In addition to chromium and molybdenum, many other elements are added to stainless steel to promote the formation of carbides, including niobium, titanium, tungsten, tantalum and vanadium. Adding niobium and titanium to austenitic stainless steel can stabilize carbon and prevent intergranular corrosion. These two elements form MC-type carbides, which do not dissolve during welding and heat treatment, thereby preventing the formation of chromium-rich M23C6-type carbides and the cause of intergranular corrosion. Tungsten, tantalum, and vanadium are added to some special stainless steel grades, mainly to form fine dispersed carbides to increase high temperature temperatures. These elements also tend to promote the formation of ferrite, because they bind the carbon, so that this powerful austenite forming element no longer plays a role, and these elements themselves also promote the formation of ferrite in the solid solution. The impact of each element is analyzed and discussed below.
1.1 Effect of Titanium on Properties of Stainless Steel and Welds
Titanium is both a carbide-forming element and a ferrite-forming element, which helps to form a single ferrite structure. Under industrial production conditions, it is impossible to completely remove interstitial elements carbon and nitrogen in stainless steel. However, very small amounts of carbon and nitrogen will also show adverse effects under certain conditions, especially in ferritic stainless steels. Adding titanium to steel can preferentially form carbides or nitrides, such as TiC, TiN, Ti (CN), etc., thereby reducing the harm caused by carbon and nitrogen. Titanium has a strong affinity with carbon and can be used to form stable titanium carbides with carbon. Such steels are called “titanium stabilized steel”. However, in the weld metal of stainless steel, titanium, unlike niobium, cannot be added as a stabilizer. Because during arc welding titanium is oxidized to a large extent during the high temperature droplet stage. Titanium also has a strong affinity for nitrogen. With the formation of TiN, adding carbon to form titanium carbonitrides Ti (CN), the formation of these nitrides is similar to titanium carbides. In the presence of Ni, titanium can form Ni3Ti compounds, of which 25% is titanium, which can cause precipitation hardening in nickel-based alloys or high-nickel alloys. Because it participates in the formation of Ni3Ti, the effect of titanium on the precipitation of the σ phase disappears. In addition, due to the presence of titanium, low-melting phases may be formed in 18Cr8Ni steel, mainly titanium carbonitrides, which begin to melt at about 1340 ° C. When the amount of titanium added is too high, the production process of steel will be deteriorated, causing a large number of surface defects, and the yield rate will be reduced, so the amount of titanium added must be limited.
1.2 Effect of niobium on the properties of stainless steel and its welds
Niobium is both a carbide-forming element and a ferrite-forming element. It can shrink the γ phase region and suppress the formation of austenite. The main difference between it and Cr and Mo is that Nb has a high affinity for C and can form stable niobium carbides. In the FeCrNi system, when the C and Nb contents cannot form niobium carbides, the influence of niobium on the γ phase is limited. The addition of niobium to the steel can preferentially form carbides or nitrides, such as NbC, NbN, Nb (CN), Fe2Nb, etc. The presence or absence of these phases is related to the composition of the steel and heat treatment conditions. In stainless steel and its weld metal, niobium and carbon can be used to form stable carbides to improve the material’s resistance to intergranular corrosion. The stable carbide formed by niobium and carbon is NbC. According to this expression, in order to fully combine the carbon atoms and niobium, the content of niobium is at least 8 times the chemical content of carbon. Under the conditions of rapid cooling during the welding process, the niobium and carbon in the molten pool do not have enough time to form a complete combination as under equilibrium conditions, so the carbon atoms cannot be completely consumed. Another important phenomenon in welding is that in the high temperature zone of the heat-affected zone, the niobium carbon compound that has been stably precipitated on the substrate or on the multi-layer weld may be re-dissolved into the base metal or the previous weld. go with. During rapid cooling after welding, the carbon dissolved by reheating can again be partially precipitated in the form of niobium carbides. When welding, the residence time is very short at high temperature, so the dissolution of niobium carbon compounds will only occur when the high temperature is higher than 1300 ° C; when the temperature is close to the solidus line (about 1430 ° C), the newly deposited welding Below or near the fusion line, a large amount of dissolution of niobium carbon compounds occurs immediately.
Another important feature of niobium is that it affects the precipitation of intermetallic phases. Except for niobium carbide and mixed carbide Fe3Nb3C, niobium carbonitride Nb (CN) and chromium niobium mixed nitride (CrNb) N are formed. Adding excess niobium, that is, the amount of niobium is more than that when it is completely combined with C and N, the Laves phase, Fe2Nb and mixed carbonitrides will be precipitated. Because C and Nb can form stable niobium carbides, the precipitation characteristics of the intermetallic phase are significantly affected by the content of C and Nb, which may reduce or completely prevent the formation of intermetallic phases. In the FeCrNi ternary system, niobium has an important effect on the precipitation of the σ phase, and its effect is similar to that of Mo. For example, when the amount of Nb is small, the precipitation of the σ phase can be promoted. However, too much Nb will form other phases, such as Laves phase, Fe2Nb and NbC. At this time, the effect of niobium on the precipitation of σ phase will be reduced or even completely lost.
The effect of niobium on the formation of low-melting phases is also important during the welding process. It can cause thermal cracks in the weld metal and heat-affected zone. Nb forms low melting point phosphides with P, Cr and Mn; Nb forms low melting point sulfur oxide inclusions with Si, Mn and Cr. In the welding of Nb-containing pure austenite CrNi cast steels with very low P and S content, the thermal cracking in the heat-affected zone is related to the content of niobium in the as-cast structure with coarse grains, which will cause low-melting Nb, The Ni phase is formed below 1160 ° C. A large amount of experience has shown that when the austenitic weld metal solidifies, the γ phase precipitates first, or in pure austenitic welds, niobium has an adverse effect on thermal cracking. If delta ferrite is precipitated once in the weld and ferrite is still contained at room temperature, the adverse effect of niobium on thermal cracking will be greatly reduced.
1.3 Effect of Vanadium on Performance of Stainless Steel and Weld
Vanadium, like titanium and niobium, is both a carbide-forming element and a ferrite-forming element. In 12% Cr martensitic stainless hot-strength steel, vanadium promotes the formation of the precipitated phase M2X, thereby making the secondary hardening effect Strengthened, as shown in Figure 18. In austenitic stainless steel, vanadium significantly improves the low temperature strength of 00Cr17Ni14Mo2N steel and keeps the impact energy of the steel above 100J. Under the aging condition, the yield strength and impact energy of this steel containing vanadium are higher than those without vanadium at 4K. In addition, vanadium-containing steel does not cause a significant change in the magnetic permeability of the steel, providing a basic guarantee for its application in the superconductor of a nuclear fusion reaction device.
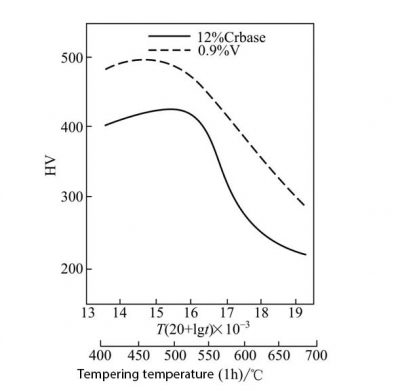
Fig. 18 Effect of vanadium on the tempering characteristics of 0.1C12Cr martensitic stainless steel (T is in K and t is in h)